I recently got an email from a reader, Stu (not his real name) who told me he got an A in Organic Chemistry 1, and was on pace to do the same in Org 2. I asked him if he’d be willing to share with the readers of this blog how he did it. I think you’ll agree that this is a story of tremendous dedication, hard work, and attention to detail. The rest I’ll leave to Stu [note: emphasis and subtitles have been added by me]
————-
How I Got An A In Organic 1
I guess I should start off with quantifying what I did in organic 1 and so far in organic 2, then I can describe how I did it.
So, this summer in organic 1 we had a quiz (mostly review stuff from gen chem, i.e. acid base chemistry, lewis structures, etc:10%), HW (25%), 2 tests (20% each) and a final (25%).
Each of the tests had 5 (out of 100) extra credit points, no extra credit for the quiz, just a hair of extra credit for the HW, which was through Sapling. The extra credit was a nice boost, making available 105 points but only grading 100 of those. So, any scores that I mention are uncurved but do include the 5 extra credit point
I got a 100% on everything except for the first exam. My initial grade was an 83%, but I submitted for a regrade and got an 87%. More on this later..
So far in Organic 2, we just took our first exam last week and I scored a 99.5%.
Now how I did it:
1. Course Preparation
I don’t really have one overarching strategy, and I wouldn’t say that my strategy is the most elegant or efficient, but I would say that I am getting there. Especially in the beginning, in was more of “siege” tactics than anything.
My one bad habit (?) with classes is getting ahead of the class pace, sometimes learning all of the subject during a semester break. So, knowing about the reputation that organic chemistry had, I decided to “run into the burning building”, for lack of better words, and tackle it head on rather than fear it. I guess you could say that my first step was to get into the right mindset.
Next, I started early, before the semester started. This way, I was able to stay about 4-5 chapters ahead of the lecture. What I would do is pre-read the chapter to know what was going on, then read it again slowly and doing all of the problems. If there was a word or concept I didn’t know, I would not move forward until I understood and mastered it. I sketched out just about every mechanism and molecule I could find. I have notebooks full of molecules and mechanisms. This was a serious time investment.
So anyway, I probably spent about 10-20 hours on each chapter, depending on the difficulty. A lot of this was time well spent, although some of it was wasted since later on in lecture the teacher would emphasis certain parts while skipping others, want us to know about certain reactions/mechanisms but not even mention others. Although my approach was not efficient, I did reach a level pretty close to mastery on those concepts once I stepped into lecture. This allowed me to fully understand everything the teacher was talking about, and to ask relevant questions.
2. Exam Preparation
About a week or two before an exam, I would take the list of “suggested book problems” that our teacher gave us and would rework those problems for the relevant chapters. Keep in mind that I had already done these problems, but by this point it was sometimes 3-4 weeks previously. Any problems I had trouble with would go onto a list and I would keep reworking them until they got eliminated from the list.
The book “Organic Chemistry as a Second Language” was a HUGE help, especially for IR and NMR. I only skimmed the textbook chapters on these subjects, as this book was more pointed and relevant.
3. Organizing Notes and Reactions
A day or two after lectures, I would compile all of my notes. Concepts would do into an outline form Word document. Reactions go into my reaction notebook. Here is a description of my reaction notebook:
4 sections:
?A chronological list of reactions with starting material, conditions, and products. Any reactions whose mechanisms were discussed in class have a *A list of mechanismsA list of reactions organized by common starting product, i.e. Reactions starting with Alkenes, starting with Primary R-OH, Secondary R-OH, Epoxides, Oxidizing Primary R-OH, etcA list of reactions organized by common ending product (retro) i.e. Reactions ending with Alkenes, Primary R-OH, Alkanes, etc
The reactions in lists 1,3,4 are all cross indexed. For example, hydroboration might be listed as #28 in each list. This makes it easy to identify errors, omissions, demo molecules that could be improved. Most of the molecules were ones given in lecture. Some of them used prototype molecules. I did this if I wanted to demonstrate a particular stereochemistry concept, or showing how different reagents lead to different products with the same molecule, etc.
My main study of reactions was with lists 3+4. I have found that identifying reactants and products is usually pretty easy if you know one or the other and the conditions, but remembering all of the conditions can be a challenge, so that is what I focused on.
4. Learning Reactions
What I would do this these lists is get a piece of paper, cover up all of the conditions, and see how many I could write out on the paper. Any that I missed would get an X next to them. I would keep going through the lists, repeating just the incorrect ones, until all of the Xs were gone. Then I would go through each group again. If I could do them all perfectly, that group got a tick mark. Then, wait a while, come back to it. If I could still do the group perfectly again, another tick mark. Once I got to 5 tick marks for each group, remembering all of the reactions was no sweat.
Me and a friend of mine traded synthesis problems for a while. I found that I learned a lot just by writing them and seeing all of the different ways I could move functional groups in 2 steps, 3 steps, etc.
This reaction notebook has been an awesome help, but unfortunately it wasn’t until I was quite a way into Organic 1 that I figured it out. Better late than never!
5. Keeping Track of Mistakes
Another thing I did was I kept a list of every mistake I did on every exam. This included conceptual, technical, and cognitive mistakes.? For example, I did horrible on my first exam, considering the amount of time I invested in it. I wasn’t lacking in the first two categories, but I made a lot of cognitive mistakes. I was a little too overconfident, so much so that I didn’t notice a lot of mistakes I made even though I checked my work a few times. Also, since I wasn’t used to how organic tests were formatted, that threw me off a little bit. I made some really dumb mistakes.
There was one mistake I remember where the question asked to outline the free radical addition of HBr to an alkene. I figured that I would be a hot shot and detail the mechanism, even though that wasn’t asked for. Also, I wasn’t entirely sure how much detail was required when it said “outline”, so I decided to play it safe and prove that I knew what I was talking about. Well, I detailed everything, except that I forgot to draw out the starting alkene!!!!!? This definitely went on the list. The moral of the story was to learn from every mistake. Also, there were a few stereochemistry concepts (particularly meso) that I still wasn’t quite strong enough on, so I had to figure those out, quickly.
6. Final Words of Advice
Another piece of advice is to not get behind and to try to understand everything as it is presented. If something looks foreign, it must be made familiar immediately. Look stuff up, do extra practice problems. I have done probably thousands of problems…way too many than I would even like to think of.
I feel like I am a little more efficient now. When I go into class, I have still read all of the chapter and have done most of the practice problems, or at least the recommended ones. Sometimes, if I don’t do all of the problems in a chapter, I will do ones that are similar to the recommended ones, since they have appeared on tests.? I don’t try to memorize every reactions before lecture, since I don’t know which ones the teacher will want us to know. Memorizing all of them would just be wasted time.
Also, I always consider homework to be “free points” in every class that I take. There is so much time and so many resources available that getting anything else than close to 100% is almost illogical to me.
Anyway, hope this helps. I think that should be the bulk of my approach.
-”Stu”
————————-
Want to share your success story (or even a failure story) ? Send me an email via the “Feedback” button above
View the original article here





 MOC is growing in traffic (yea!) but that means that the hamsters inside my current server are running at the brink of exhaustion. I’m migrating/upgrading to a new server service, so no posts for a few days until this gets settled. Hopefully by next week page load times should be snappier too.
MOC is growing in traffic (yea!) but that means that the hamsters inside my current server are running at the brink of exhaustion. I’m migrating/upgrading to a new server service, so no posts for a few days until this gets settled. Hopefully by next week page load times should be snappier too.







 Early morning in Jerusalem, 2010
Early morning in Jerusalem, 2010


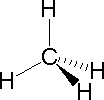
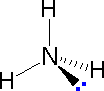
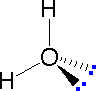
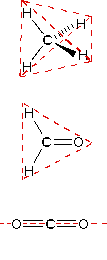
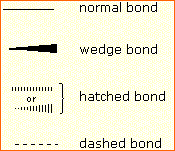 In order to represent such configurations on a two-dimensional surface (paper, blackboard or screen), we often use perspective drawings in which the direction of a bond is specified by the line connecting the bonded atoms.
In order to represent such configurations on a two-dimensional surface (paper, blackboard or screen), we often use perspective drawings in which the direction of a bond is specified by the line connecting the bonded atoms.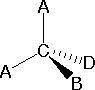 In most cases the focus of configuration is a carbon atom so the lines specifying bond directions will originate there. As defined in the diagram on the right, a simple straight line represents a bond lying approximately in the surface plane. The two bonds to substituents A in the structure on the left are of this kind. A wedge shaped bond is directed in front of this plane (thick end toward the viewer), as shown by the bond to substituent B; and a hatched bond is directed in back of the plane (away from the viewer), as shown by the bond to substituent D. Some texts and other sources may use a dashed bond in the same manner as we have defined the hatched bond, but this can be confusing because the dashed bond is often used to represent a partial bond (i.e. a covalent bond that is partially formed or partially broken). The following examples make use of this notation, and also illustrate the importance of including non-bonding valence shell electron pairs (colored blue) when viewing such configurations .
In most cases the focus of configuration is a carbon atom so the lines specifying bond directions will originate there. As defined in the diagram on the right, a simple straight line represents a bond lying approximately in the surface plane. The two bonds to substituents A in the structure on the left are of this kind. A wedge shaped bond is directed in front of this plane (thick end toward the viewer), as shown by the bond to substituent B; and a hatched bond is directed in back of the plane (away from the viewer), as shown by the bond to substituent D. Some texts and other sources may use a dashed bond in the same manner as we have defined the hatched bond, but this can be confusing because the dashed bond is often used to represent a partial bond (i.e. a covalent bond that is partially formed or partially broken). The following examples make use of this notation, and also illustrate the importance of including non-bonding valence shell electron pairs (colored blue) when viewing such configurations .